Page 45 - รายงานประจำปี ๒๕๕๕ ศูนย์องค์รวมเพื่อการศึกษาและบำบัดโรคมะเร็ง คณะแพทยศาสตร์
P. 45
รายงานประจ�าปี 2555
ศูนย์องค์รวมเพื่อการศึกษาและบ�าบัดโรคมะเร็ง
QUALITY AND ACCESSIBILITY IMPROVEMENT OF
HER2 FLUORESCENCE in situ HYBRIDIZATION
(FISH) DIAGNOSIS BY AN IN-HOUSE DUAL-COLORED
PROBE SET
Phatcharaporn Thongwatchara 1 , Hatairat Hongphruk 1 , Sukanya Meesa 4 , Pleumjit Boonyaphiphat 2 , Arunee Dechaphunkul 1 , Sinitdhorn
Rujirabanjerd 2 , Somkiat Sunpaweravong 3 , Puttisak Puttawibul 3 , Verayuth Praphanphoj 4 , Patrapim Sunpaweravong 1 .
1 Holistic Center for Cancer Study and Care (HOCC-PSU), Division of Medical Oncology, 2 Department of Pathology, 3 Department of
Surgery, Faculty of Medicine, Prince of Songkla University, Songkhla. 4 Rajanukul Institute, Ministry of Public Health, Bangkok. Thailand.
Abstracts
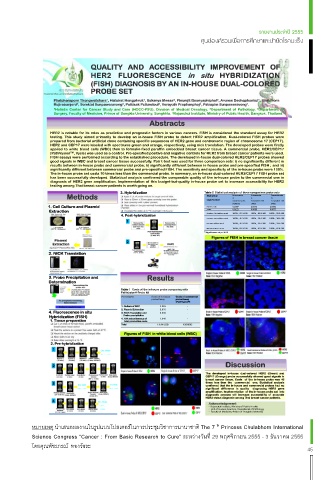
HER2 is notable for its roles as predictive and prognostic factors in various cancers. FISH is considered the standard assay for HER2
testing. This study aimed primarily to develop an in-house FISH probe to detect HER2 amplification. Dual-colored FISH probes were
prepared from bacterial artificial clone containing specific sequences of HER2 gene and centromeric region of chromosome 17 (CEP17).
HER2 and CEP17 were labeled with spectrums green and orange, respectively, using nick translation. The developed probes were firstly
applied to white blood cells (WBC) then to formalin-fixed paraffin embedded breast cancer tissue. A commercial probe, HER2/CEP17
(PathVysion TM , Vysis) was used as a control. Pre-specified positive and negative controls for HER2 from breast cancer patients were used.
FISH assays were performed according to the established procedure. The developed in-house dual-colored HER2/CEP17 probes showed
good signals in WBC and breast cancer tissue successfully. Pair t-test was used for three comparison sets: i) no significantly different in
results between in-house probe and commercial probe; ii) significantly different between in-house probe and pre-specified FISH ; and iii)
significantly different between commercial probe and pre-specified FISH. The sensitivity and specificity of the in-house probe were 100%.
The in-house probe set costs 10 times less than the commercial probe. In summary, an in-house dual-colored HER2/CEP17 FISH probe set
has been successfully developed. Statistical analysis confirmed the comparable quality of the in-house probe to the commercial one in
diagnosis of HER2 gene amplification. Implementation of this budget-but-quality in-house probe set to increase accessibility for HER2
testing among Thai breast cancer patients is worth going on.
3. Hybridization Table 2 Statistical analysis of three comparison probe sets
Methods Statistical In-house probe In-house probe Commercial probe
significance vs Commercial probe vs Pre-specified FISH Vs Pre-specified FISH
(PathVysion) (DAKO) (DAKO)
1. Cell Culture and Plasmid Pair t-test, 2 tails 0.2397 2.8173 2.8781
Extraction p-value 0.8123 0.0089 0.0077
4. Post-hybridization Specificity , 95% Confidence interval 100% , 83.4-100 100% , 82.8-100 100% , 82.8-100
Sensitivity , 95% Confidence interval 100% , 46.3-100 100% , 39.6-100 100% , 39.6-100
1
PPV, 95% Confidence interval 100% , 46.3-100 100% , 39.6-100 100% , 39.6-100
NPV, 95% Confidence interval 100% , 83.4-100 100% , 82.8-100 100% , 82.8-100
* Significance at p < 0.05
Figures of FISH in breast cancer tissue
2
2. NICK Translation
3. Probe Precipitation and Results
Determination
Table 1 Costs of the in-house probe comparing with
PathVysion® Probe kit
Procedures Costs of in-house Costs of commercial
probe/Assay probe/Assay
(PathVysion ® )
1. Culture of BAC 0.825 -
2. Plasmid Extraction 2.970 -
4. Fluorescence in situ 3. NICK Translation and 6.605 -
Hybridization (FISH) Probe precipitation
1. Tissue preparation 4. 10% miscellaneous of 1.040 -
total production costs
Total 11.44 USD 126 USD
Figures of FISH in white blood cells (WBC)
2. Pre-hybridization
1
2 Discussion
The developed in-house dual-colored HER2 (Green) and
CEP17 (Orange) probe successfully showed good signals in
breast cancer tissue. Costs of the in-house probe was 10
3 times less than the commercial one. Statistical analysis
significant difference in quality diagnosing HER2 gene
confirmed that the in-house and commercial probes had no
amplification. Implementation of the in-house probe set into
diagnostic process will increase accessibility of accurate
HER2 status diagnosis among Thai breast cancer patients.
4
Acknowledgement
• Rajanukul Institute, Ministry of Public Health
• Unit of Human Genetics, Department of Pathology
• Faculty of Medicine, Prince of Songkla University
หมายเหตุ น�าเสนอผลงานในรูปแบบโปสเตอร์ในการประชุมวิชาการนานาชาติ The 7 Princess Chulabhorn International
th
Science Congress “Cancer : From Basic Research to Cure” ระหว่างวันที่ 29 พฤศจิกายน 2555 - 3 ธันวาคม 2555
โดยคุณพัชรภรณ์ ทองวัชระ 45